Engineering heart muscle, healing humans: Stuart Campbell’s biomedical vision
Written by Natalie Haase '27
Some scientists fall into their field by accident. For Stuart Campbell, it was more like gravity. As an undergraduate, he became captivated by the heart — not for its symbolic meaning, but for its mechanics. “It moves,” he said simply. “It contracts on its own. That’s just fascinating.” Campbell joined the Yale faculty and launched his lab in July 2012, just as CRISPR/Cas9 gene-editing technology was emerging and beginning to revolutionize the study of genetic disease. The timing was serendipitous: the power to precisely edit the genome became central to the way Campbell and his team investigate inherited heart conditions at the molecular level.
Read more • Approximately 4 minutesToday, Campbell is a professor of biomedical engineering at Yale, where he leads a lab that engineers human heart tissues to understand how genetic mutations lead to disease. His work centers on cardiomyopathies, conditions where the heart muscle becomes abnormally thickened or dangerously thin and weak — often due to a single error in a gene. “If we can understand heart muscle in a quantitative way,” Campbell says, “we should be able to propose ways of fixing it.”
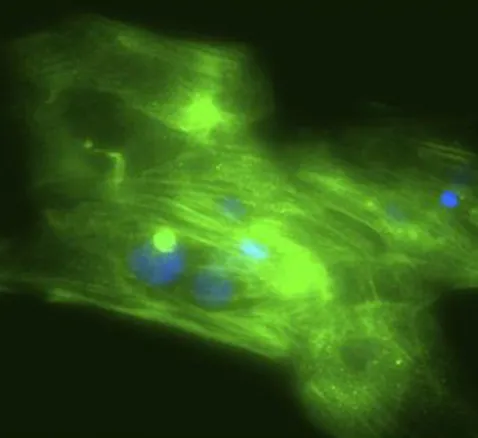
In a recent study, the Campbell Lab used CRISPR/Cas9 to insert different mutations into human-induced pluripotent stem cells. One mutation caused hypertrophic cardiomyopathy — a thickened, overactive heart wall that contracts too forcefully and increases the risk of arrhythmia. The other led to dilated cardiomyopathy, where the heart wall thins, weakens, and struggles to pump blood effectively. Both mutations originated in the same gene, which codes for tropomyosin, a molecular switch that helps regulate whether heart muscle cells are “on” or “off” with each beat. In a controlled lab setting, Campbell’s team grew strips of engineered heart tissue (EHT) from these edited stem cells and observed the muscle behavior. The dichotomy was striking: the same gene, with just a different substitution, sent the heart down two opposite and dangerous paths.
““It’s incredibly motivating. This is a disease that affects people in the prime of their lives — when they’re raising kids, taking care of others, working hard to do good in the world. Being able to work on something that directly affects them is a privilege.”
Stuart Campbell
Professor of Biomedical Engineering and Cellular and Molecular Physiology
While many labs grow engineered heart tissue by combining cardiac cells with hydrogels and casting them into molds, Campbell’s team took a different approach. They start with a thin slice of decellularized pig heart tissue — essentially, the natural scaffold of an organ stripped of its cells — and use that as a foundation. Human heart cells, derived from stem cells, are then seeded onto the matrix. With mechanical stimulation and hormone cues, the cells organize and mature, beating rhythmically and mimicking the behavior of human muscle. “It’s like a treadmill for tissues,” Campbell says. “We can mimic developmental stages, like the surge in hormones that happens after birth, to push these cells toward more adult-like function.”
This process — layering genome editing, stem cell reprogramming, and biomechanics — allows the lab to not only model disease but to test how different conditions might be treated or even prevented. If built correctly and maintained in the right conditions (media, glucose, pH, ion balance), these tissues can survive indefinitely. “We’ve been all about mechanism up until this point,” Campbell explains. “Now we’re asking how to use that understanding to test therapies — drug treatments, genome correction, anything that might make a difference.”

For Campbell, the work is personal. Long after his scientific interest in heart muscle had taken root, his life intersected with a close family connection to inherited cardiomyopathy. That link deepened his investment in the work. He’s since helped family members map their genetic risk, genotyped generations at reunions, and worked to understand the mutation’s clinical implications. “It’s incredibly motivating,” he says. “This is a disease that affects people in the prime of their lives — when they’re raising kids, taking care of others, working hard to do good in the world. Being able to work on something that directly affects them is a privilege.”
That sense of purpose also carries into how he teaches and mentors. Campbell sets aside every Wednesday to meet one-on-one with his students — a space for collaborative science, where ideas are discussed with patience and excitement. “It’s a blast,” he says. “These students are brilliant. It’s so fulfilling to be part of their development as scientists.”
When he’s not guiding research or decoding genetic mechanisms, Campbell is building in a different sense: at home, he’s a father of four and a weekend woodworker. Recent projects include custom-built bunk beds for his two sons.
It’s not a bad metaphor for his science. Whether in the lab or in life, Campbell builds with intention: structural precision in service of human wellbeing.
More Details
Published Date
Jul 15, 2025